VasaPlex is a biologic drug developed by Samba BioLogics that saves tissue after heart attack.
VasaPlex (HGF:IgG complex) and VasaPlex-F2 (FGF2:HGF:IgG complex) are vaso- and cardioprotective biologic drugs we’ve designed to increase vascular integrity and preserve cardiac tissue jeopardized by reperfusion injury and low/no-re-flow.
About Our Solutions
VasaPlex
Coronary heart disease leading to acute myocardial infarction (MI; heart attack) is a principal cause of mortality worldwide. Cornerstone treatments for MI are designed to restore blood flow (i.e. to “reperfuse”) blocked coronary arteries. Despite reduced times to intervention, and successful stent placement, 30-50% of primary Percutaneous Coronary Intervention (PCI) patients exhibit low- or “no-reflow”, a phenomenon linked to poor outcomes, increased probability of heart failure, and death. Low/no-reflow occurs when macroscopic vessels are opened by stenting or thrombolysis, but distal myocardial perfusion remains compromised. Notably, even if they do not exhibit low- or no-reflow, nearly all patients will experience reperfusion injury after MI and PCI w/stenting.
Samba BioLogics, Inc., is providing new, innovative and effective products to improve patient survival, outcomes and quality of life after MI. In a large animal (pig) model of acute MI with reperfusion, intracoronary infusion of VasaPlex preserved 48 ± 12% of myocardial tissue at risk. At 24 hours after M, treatment with VasaPlex-F2 preserved epicardial electrical conduction across the region with infarction.
VasaPlex cuts heart attack injury in half
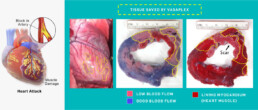
- Pre clinical large animal model of MI w/24 hr reperfusion (adult female pigs)
- PCI catheters/stents from UVM Medical Center (same used in patients with MI)
VasaPlex Achievements
Defined
Mechanism of action in rodents w/MI
Preclinical Testing
Adult pigs with MI
Awarded
Samba BioLogics, NIH STTR $500k
Developed
VasaPlex F2 Platform
Awarded
NIH/NHLBI RO1 $1.4M
Strong Clinical Feasibility
Requires only 2 minute infusion from indwelling PCI guide catheter after stenting
Determined
No risk of autoantibodies (safety)

